Being a remote chemist in Jet City, aka the land of BAMS*, an obvious point to take off for chemical connections is the University of Washington. I found the chemistry departments events page online and headed to a seminar for the first time in many years. The campus sparkles in the Seattle sunshine and walking through the blooming Sakura down the hill to the big fountain, Mt. Ranier peaks above the trees. A quick summary of the seminar is below, but first two non-science take-aways 1) after so many 15min conference presentations, its really hard to pay attention for a full hour. 2) no cookies? Is that only done in Westwood? Shout out to Diddy Riese.
P Shing Ho of CSU presented a very cool talk about biological halogen bonds, BXBs. Through some observational serendipity working on Holliday Junctions, Ho became interested in the non-covalent binding potential of halogens. While most molecular simulation programs have characterized halogens as spheres of electronic density, they do have sigma holes. A more accurate image would be a sphere where the top portion has small positive charges and can non-covalently bond with negative charges just like a hydrogen atom. Like if the Earth were a giant Br , the arctic circle would be sigma positive.
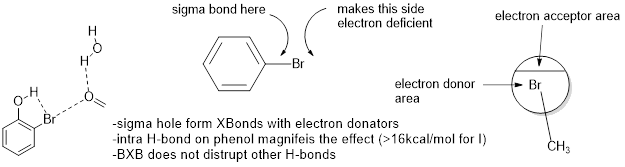
But wait there’s more! This BXB can be formed EVEN WHERE THERE IS AN EXISTING H-Bond. So halogenated phenols, with an intramolecular H-bond to the halogen, make the donut hole even bigger! Check out this paper.
*Boeing Amazon Microsoft Starbucks
